Increasing Energy Storage in Electrochemical Capacitors with Ionic Liquid Electrolytes and Nanostructured Carbon Electrodes
Jenel Vatamanu , Zongzhi Hu , and Dmitry Bedrov , Department of Materials Science & Engineering, University of Utah, 122 South Central Campus Drive, Room 304, Salt Lake City, Utah 84112, United States
Carlos Perez and Yury Gogotsi Department of Materials Science & Engineering, and A.J. Drexel Nanotechnology Institute, Drexel University, 3141 Chestnut Street, Philadelphia, Pennsylvania 19104, United States
J. Phys. Chem. Lett., 2013, 4, pp 2829–2837 DOI: 10.1021/jz401472c Publication Date (Web): August 7, 2013 Copyright © 2013 American Chemical Society
Abstract
 The potential pathways to increase the energy storage in electric double-layer (EDL) supercapacitors using room-temperature ionic liquid electrolytes and carbon-based nanostructured electrodes are explored by molecular dynamics simulations. A systematic comparison of capacitances obtained on nanoparticles of various shape and dimensions showed that when the electrode curvature and the length scale of the surface roughness are comparable to ion dimensions, a noticeable improvement in the capacitive storage is observed.
The potential pathways to increase the energy storage in electric double-layer (EDL) supercapacitors using room-temperature ionic liquid electrolytes and carbon-based nanostructured electrodes are explored by molecular dynamics simulations. A systematic comparison of capacitances obtained on nanoparticles of various shape and dimensions showed that when the electrode curvature and the length scale of the surface roughness are comparable to ion dimensions, a noticeable improvement in the capacitive storage is observed.
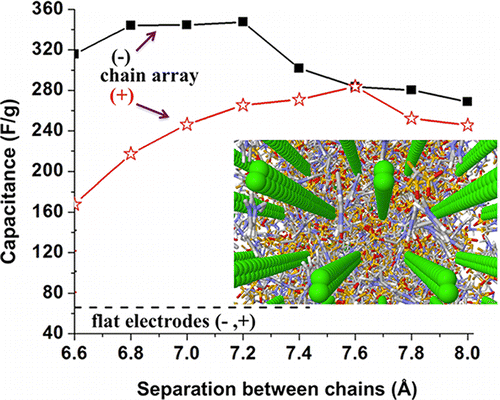
The nanoconfinement of the electrolyte in conductive electrode pores further enhances the capacitance due to mismatch in ion–electrode surface interactions and strong electrostatic screening. We show that nanoporous structures made of arrays of conductive carbon chains represent a synergy of all three favorable factors (that is, high curvature, atomic scale roughness, and nanoconfinement) and can generate non-Faradic capacitance ranging from 260 to 350 F/g, which significantly exceeds the performance of the current generation of nanostructured electrodes.
Supplementary Information
Ïîäðîáíåå íà ñàéòå www.mrc.org.ua