
The 224th ECS Meeting in San Francisco, California | October 27 – November 1, 2013
The 224th Electrochemical Society ECS Meeting will be held in the heart of San Francisco, at the meeting headquarters hotel, the Hilton San Francisco (333 O’Farrell Street, San Francisco, CA 94102).
This major international conference at the Hilton San Francisco will include more than 50 topical symposia consisting of over 2,800 technical presentations, and feature the third international ECS Electrochemical Energy Summit (E2S), which is fast becoming a tradition at ECS meetings. 2S and ECS Short Courses help launch the meeting on Sunday, October 27.
On Wednesday, October 30, 2013 prof. Yury Gogotsi will report on High Electrosorption Capacity Electrodes for Capacitive Deionization at the Energy–Water Nexus Symposium (A3) of ECS Electrochemical Energy Summit, at 224th ECS Meeting.
High Electrosorption Capacity Electrodes for Capacitive Deionization
Kelsey B. Hatzell1, Etsuro Iwama2, Barbara Daffos2, Pierre-Louis Taberna2, Theo Tzedakis2, Alexei Gogotsi3 , Patrice Simon2, Yury Gogotsi1
1 A.J. Drexel Nanotechnology Institute, Materials Science and Engineering Department, Drexel University, Philadelphia, PA
2 Université Paul Sabatier, CIRIMAT UMR CNRS 5085, 118 route de Narbonne, 31062 Toulouse, France
3 Materials Research Centre, 03680 Kiev, Ukraine
Abstract
In water stressed regions across the globe, the rate of abstraction from deep aquifers often exceeds the rate of recharge. This leads to water shortages that are sometimes irreversible. In order to address these water shortages, researchers are looking to the most abundant of source water present on earth, seawater. However, to transform seawater into clean drinking water requires a range of energy intensive processes. Such processes include Reverse Osmosis, UV disinfection and Thermal Distillation. The most promising of these technologies is Reverse Osmosis, which can achieve 1.8 kWh/m3 in current commercial plants [1]. Nevertheless, this technology is fundamentally hindered by membrane fouling and slow water transport [2]. Thus, there has been a movement toward technologies that do not use membranes, and toward technologies that remove the minority component (salt) rather than the majority component (water) [3].
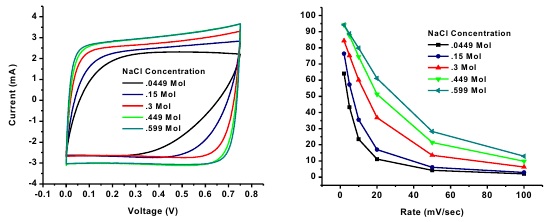
Figure 1. (a) Cyclic voltammetry performance of spherical activated carbon based electrodes in different NaCl solutions at 2 mV s-1. (b) Rate performance of CDI electrodes in different NaCl concentrated solutions.
Capacitive Deionization (CDI) is the process of removing ions from brackish/seawater by applying a potential between two electrodes, adsorbing ion on the surface, and producing clean water. Carbon materials are favorable as electrode materials in CDI systems because they exhibit high electric conductivity (~100 S m-1), specific surface area (up to 2000 m2 g-1), and high electrochemical stability. Herein, we report the use of spherical activated carbon beads (BET SSA 1219 m2 g-1) as the active material for electrodes for a capacitive deionization system. In a 0.15 M solution of NaCl at 10 mV s-1 the electrodes demonstrate a capacitance of 58 F/g which is on par with recently reported electrode capacitances. These results indicate that with further optimization, the spherical geometry of the particles may yield enhanced electrosorption capacity for CDI.